Effects of electrical stimulation on WGA-HRP transport in pubococcygeus motoneurons of male rats
- Inicio
- Comité Editorial
- Lineamientos
- Carta de Cesión de Derechos
- Información Legal
- Acerca de la Revista
- Bases de Datos
- Contacto
- ISSN 2007-3054
- Centro de Investigaciones Cerebrales
Universidad Veracruzana
Artículo de Investigación
Efectos de la estimulación eléctrica sobre el transporte de WGA-HRP en motoneuronas del pubococcígeo de ratas macho
Cesar Antonio Perez1*, Maria del Carmen Aguirre1, Eduardo Chang1, Genaro A. Coria-Avila1+, Leonor Lopez-Meraz1*, Consuelo Morgado1*, Luis Beltran1*, Rolando Garcia-Martinez3, Maria Elena Hernandez1**, Luis Isauro Garcia1+, Porfirio Carrillo2 and Jorge Manzo1+
1Programa de Neurobiología, Universidad Veracruzana, 2Instituto de Neuroetología, Universidad Veracruzana, 3Laboratorio de Neurobiología, Universidad Autónoma de Campeche, Camp., México, *Cuerpo Académico de Neurofisiología del Programa de Neurobiología. +Cuerpo Académico de Neurociencias, **Cuerpo Académico de Neuroquímica.
Abstract/Resumen
Introduction
Materials and Methods
Results
Discussion
Conclusions
Acknowledgements
References
Corresponding Author
Introduction: The retrograde axonal transport from dendrites and the axon terminal to the soma is very important mechanism for transferring contents among the different regions of the neuron. However, the correlation between tracers labeling on motoneurons with neural electrical activity is somewhat debatable.
Objetives: Therefore, we used horseradish peroxidase conjugated to wheat germ agglutinin (WGA-HRP) to analyze spinal motoneurons of the Pubococcygeus (Pc) muscle in male rats that received electrical stimulation on the left somatomotor branch of the pelvic nerve.
Materials and Methods: Three groups were made according to the timing of stimulation: A) after injection of WGA-HRP, B) before perfusion of the rat, and C) both conditions, A+B.
Results: The results indicated that motoneurons located on the ipsilateral side of stimulation applied twice (group C) obtained the lower values of morphological labeling.
Conclusions: We suggested that the electrical activity of a motoneuron results in an increase of dendritic movement to concentrate substances in the soma, which indeed regulates WGA-HRP labeling. It is suggested that the state of electrical activity of a motoneuron increases the centripetal movement of cytoplasmic contents, as represented by the stain. The mechanism could serve to maintain the integrity of the different regions of the motoneuron by allowing recycling, degradation or transferring of components into the soma.
Palabras clave: Nerve stimulation, motoneurons, WGA-HRP.
Introducción: El transporte axonal retrógrado desde las dendritas y de terminales axónicas hacia el soma es un mecanismo importante para obtener información del cuerpo celular a las dendritas y terminales nerviosas. La correlación entre el marcaje de trazadores sobre motoneuronas con la actividad eléctrica neuronal es algo debatible.
Objetivos: Por lo tanto, usamos la peroxidada de rábano conjugada con germen de trigo aglutinado (WGA-HRP) para analizar motoneuronas espinales del músculo Pubococcígeo (Pc) en ratas macho que recibieron estimulación eléctrica de la rama somatomotora izquierda del nervio pélvico.
Material y Métodos: Tres grupos fueron usados de acuerdo al tiempo de estimulación: A) después de la inyección de la WGA-HRP, B) antes de la perfusión de la rata, C) ambas condiciones, A+B.
Resultados: Los resultados indicaron que las motoneuronas localizadas del mismo lado de la estimulación aplicada dos veces (grupo C) obtuvieron los valores más bajos del marcaje morfológico.
Conclusiones: Sugerimos, que la actividad eléctrica de una motoneurona resulta en un aumento del movimiento dendrítico para concentrar substancias en el soma, el cual realmente regula el marcaje de la WGA-HRP. Esto sugiere que estado de la actividad eléctrica de una motoneurona aumenta el movimiento centrípeto del contenido citosplásmico, representado por la tinción. El mecanismo podría servir para mantener la integridad de regiones diferentes de la motoneurona para permitir el reciclaje, la degradación o la transferencia de componentes al soma.
Keywords: Estimulación nerviosa, motoneuronas, WGA-HRP.
The Pubococcygeus muscle (Pc) is located in the pelvic floor of both males and females, and it is innervated by the somatomotor branch of the pelvic nerve, which originates between the sixth lumbar and the first sacral segments of the spinal cord in rats.1, 2 It has been demonstrated that the Pc participates in reproductive processes and micturition. In male rats for example, denervation of the Pc after cutting the somatomotor branch of the pelvic nerve produces the deposit of smaller sperm plugs during ejaculation, and those males fail to impregnate females compared with intact males. Furthermore, as observed with electrophysiological studies, the Pc muscle responds actively with contractions during micturition.3
The motoneurons that innervate the Pc muscle are sensitive to the fluctuation of systemic hormones. Specifically, there is evidence that in both males4 and females5 gonadectomy reduces some morphological patterns of the motoneurons such as the soma area, the length of primary dendrites and the dendritic arborization area, as observed with horseradish peroxidase labeling conjugated to wheat germ agglutinin (WGA-HRP). In male rats, the effect of castration is reversed by subcutaneous testosterone, which restores the morphological patterns after four weeks of treatment.4 Interestingly, castrated males treated with estradiol benzoate recover the morphological patterns within two weeks of treatment, indicating that the aromatization of testosterone is an important physiological process for the normal function of Pc motoneurons. However, the spinal cord of male rats does not show detectable aromatase activity,6 which has lead us to suggest that estrogens influence motoneurons via supraspinal neurons.4
It has been reported that neurons of the Paraventricular Nucleus (PVN) of the hypothalamus project to the lumbosacral spinal cord,7-9 and therefore could modulate activity in motoneurons of muscles of the pelvic floor important for the expression of penile erection and sexual behavior.10-13 The PVN has estrogen receptors and aromatase activity,14 and we have previously demonstrated that local treatment with blockers for androgens, estrogens and aromatase within the PVN, as well as spinal cord transection, produce an effect similar to castration on Pc motoneuronas.15 Given that there are no estrogen receptors in the lumbosacral segments in males rat, the effects of these hormones on Pc motoneurons are believed to be mediated via other neurotransmitters, such as oxytocin (OT) at the level of the dorsal gray commissure, the intermediomedial gray matter, and the sacral parasympathetic nucleus.13,16-17 In fact, the effects of spinal cord transection are reversed with intrathecal injections of oxytocin in the lumbosacral segments, since oxytocin-treated animals recovered morphological features similar to those of intact animals.15
It has been accepted to some extent that the morphological changes observed with WGA-HRP in the motoneurons during the fluctuation of steroids hormones are due to the increase or reduction of intracellular transport of the tracer and not due to changes in the size of neurons.18 Those changes are believed to be an objective measure of the activity and sensitivity of motoneurons to physiological changes. Accordingly, it is possible that the hormonal fluctuation and other stimuli such as electrical stimulation may affect the intracellular transport of substances. Thus, it is not clear yet whether the HRP retrograde labeling is really correlated with the neural activity in the mammalian central nervous system.
Nishino and colleagues19, for example, demonstrated that stimulation of the sciatic nerve 1 hour by during the survival period increased the numbers of motoneuros of the Medial Gastrocnemius muscle (MG) labeled retrogradely with HRP, as compared to non-stimulated (control group) in rats.19 This study suggested that retrograde transport of HRP in the gastronecmius-sciatic system depends on the activity of both nerve and muscle. It decreases when nerve and muscle activity is low and increases when their activity is high. In contrast, Sato en colleagues showed that there were no significant difference in the numbers of labeled cells between the stimulated and the non-stimulated sides of rats. Thus, it seems that the electrical stimulation of the sciatic nerve did not affect HRP retrograde labeling of MG motoneurons of the rat.20 In adittion, a study driven by Kanda and colleagues,21 there were no significant differences in the number of labeled hindlimb motoneurons between the blocked and intact sides of rats.
There are several limitations to the previous studies of these authors. For example, these studies used HRP alone and not combined with wheat germ agglutining (WGA). It has been shown that HRP alone is transported more slowly than when combined with WGA.22 In addition, these studies differed in the amount of time allowed for the HRP to be transported since the moment of injection. Specifically, it has been shown that the appropriate time for the tracer to travel from the axon terminals to the soma of motoneurons that innervate to pelvic muscles is about 48 hrs.4, 23-25 Consequently, it is possible that less time, plus the use of HRP alone may have not been sufficient for the tracer to reach the soma and dendrites of motoneurons that innervate hindlimb muscles. Unfortunately, the authors did not consider more variables to assess intracellular distribution of the tracer.
Thus, given that the effects of electrical stimulation on the number of motoneurons appear to be ambiguous, the present study was designed to investigate whether electrical stimulation applied to the somatomotor branch of the pelvic nerve would affect the retrograde labeling of WGA-HRP in Pc motoneurons, mainly in morphological characteristics more detailed such as the soma area, the length of primary dendrites and the dendritic arborization area.
2.1 Animals
Adult Wistar male rats (Harlan Laboratories, México, D.F.) with a body weight between 250-350 g were housed in groups of three in plexiglass shoe boxes on a 12:12-h light/dark cycle and were given free access to Purina rat chow and water.
The experiments required the animals to undergo a surgery during which injection of the neural tracer was administered directly into the Pc muscle. Every surgery and manipulation of the rats was guided by the Policy’s Manual on the Use of Animals in Neuroscience Research of the Society for Neuroscience.
2.2 Experimental procedure
Twenty-four males received bilateral injections of WGA-HRP directly into the Pc muscle, and were sacrificed 48 hr later. The animals were organized in three groups (n=8), which received unilateral electrical stimulation on the somatomotor branch of the pelvic nerve, applied as follows: group A) immediately after the injection of WGA-HRP, group B) 15 min before the sacrifice, and group C) a combination of A+B (i.e., stimulation after injection of the tracer and 48 hr later just before perfusion of the rat). Given that electrical stimulation was applied exclusively in the left side of each animal, the right side was used as a within-groups comparison to assess the effects of both ipsilateral and contralateral electrical stimulation on the motoneurons labeling.
2.3 WGA-HRP injection in the Pubococcygeus muscle
The rats were anesthetized with an intraperitoneal injection of sodium pentobarbital (30 mg/kg bw), and placed in dorsal position. The abdomen was shaved disinfected, and a midline incision was performed with a scalpel. The intestines were gently retracted rostrally, and the bladder and seminal vesicles were retracted caudally to allow the visualization of the region where the Pc originates. Under a Leica surgical microscope, the muscle was identified and injected slowly (2-3 min) with 25 μg of WGA-HRP previously diluted in 5 μl of 1% dimethyl sulfoxide (Sigma-Aldrich).
2.4 Electrical stimulation of the somatomotor branch of the pelvic nerve
The left somatomotor branch of the pelvic nerve was identified as in a previous study by Lucio et al.,1 and was stimulated according to the groups mentioned above. A bipolar silver chloride electrode was used for stimulation. It was plugged into a stimulus isolation unit (SIU5, Grass instruments, USA), that was driven by a square pulse stimulator (S48, Grass instruments, USA). Stimuli were applied at 1 Hz and 0.1 ms of duration, starting at zero voltage and were increased slowly. Stimulus threshold was determined when contractions of Pc muscle were observed under a stereomicroscope (Leica GZ6). The stimulator was set to deliver 2-4 threshold stimuli during 15 min to the somatomotor branch of the pelvic nerve. Following the injection procedure and electrical stimulation, the viscera were put back into the cavity, and the muscle and the skin were sutured. The animals were placed in the warm chamber until they recovered from the anesthesia, and received sodium metamizol (130 mg/kg) for postsurgical analgesia and benzatinic penicillin (40 000 UI/rat) to prevent infections.
2.5 Technique of WGA-HRP labeling in Pc motoneurons
Forty-eight hours later, the males were anesthetized again with a higher dose of sodium pentobarbital (50 mg/kg bw), and were immediately perfused transcardially with 500 ml of heparinized saline solution, 800 ml of fixative at room temperature (1% paraformaldehyde and 1.25% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4) and with 600 ml of 10% cold sucrose (4°C) in 0.1 M phosphate buffer, pH 7.4 for cryoprotection. The lumbosacral spinal cord was removed, and a longitudinal and superficial cut was done on the dorsal part of the non-stimulated side to be used as a landmark to differentiate between right and left side. Then, the spinal segment was immersed in another cold sucrose-buffer solution for a period of 72 hours before being cut, and sectioned transversally (40 μm of thickness) on a Leica cryostat. Cord slices were immersed in fresh phosphate buffer without sucrose and kept at 4°C during 24 h, and reacted according to the tetramethylbenzidine method to produce dark-blue staining of the motoneuronas.25-26 The slices were mounted on gelatinized slides, dehydrated, cleared, and counterstained with 1% neutral red heated at 50-55°C to produce a reddish background, and then coverslipped with undiluted permount.
2.6 Image Processing and Statistical Analysis
Digital photomicrographs of motoneurons were obtained by using an Olympus Provis AX-70 microscope connected to a CoolSnap-Pro video camera (Media Cybernetics), and images were processed with a Flashpoint 128 frame grabber (Integral Technologies) inserted in a Dell Pentium computer. The Image-Pro Plus software (Media Cybernetics) was used to obtain the morphometric analysis of motoneurons. The parameters were analyzed as follows: 1) soma size, which was obtained with outline drawings of the labeled cell body, 2) the dendritic arborization area, measured by anchoring points at the tip of each labeled dendrite in a motoneuron with the computer mouse, and 3) length of primary dendrites, measured by drawing a single line over the dendrite image with the mouse. Morphometric measures were only obtained from motoneurons that were stained alone and showed a clear and easily distinguishable shape, and not from those located in clusters that were difficult to evaluate. Data were analyzed with a mixed design (between and within) ANOVA with the Statistica software. All significant differences were confirmed by a Tukey posthoc analysis and the level of significance was set at p<0.05.
In all the individuals, WGA-HRP labeling was observed in the Pc motoneurons, which were located in the central region of the Lamina IX of the sixth lumbar and first sacral spinal cord segments (Fig. 1). The results about the three different morphometric features are as follows:
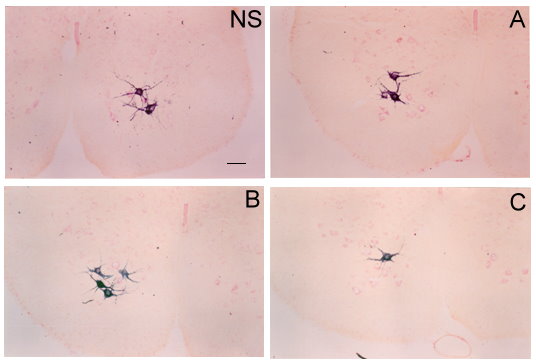
Figure 1. Photomicrographies of transverse sections of Pubococcygeus motoneurons labeled with WGA-HRP in the non-stimulated (NS) and stimulated side (A, B, and C). Scale bar = 100 µm.
3.1 Soma area
The ANOVA revealed a main effect of electrical stimulation F(1,23)= 11.8, p= 0.002, indicating that overall, the soma area of the motoneurons located in the stimulated side was smaller than those located in the non-stimulated side. The post hoc analysis, however, indicated that such differences were statistically significant only in group C (M= 1303.69 ± 117.82 μm2 and M= 991.37 ± 40.18 μm2 for non-stimulated and stimulated sides, respectively), but not in group A (1069.58 ± 41.91 μm2 and 928.12 ± 56.02 μm2 for non-stimulated and stimulated sides) or B (1159.62 ± 59.31 μm2 and 1020.00 ± 58.08 μm2 for non-stimulated and stimulated sides, respectively). The ANOVA failed to detect significant differences between groups F(2,23)= 2.7, p= 0.08, or an interaction between side of stimulation and group F(2,23)= 0.99, p= 0.38 (Fig. 2).
3.2 Length of primary dendrites
The ANOVA detected a main effect of electrical stimulation F(1,23)= 19.6, p= 0.0002, and a main effect of group F(2,23)= 9.8, p= 0.0009. However, there was no interaction between side of stimulation and group F(2,23)= 0.18, p= 0.82. The post hoc analysis failed to detect significant differences within groups A (67.86 ± 1.42 μm and 50.00 ± 4.15 μm for the non-stimulated side and the stimulated side, respectively), B (65.04 ± 3.62 μm and 46.87 ± 6.67 μm for non-stimulated and stimulated side, respectively), or C (48.45 ± 3.48 μm and 35.25 ± 4.86 μm for non-stimulated and stimulated side, respectively). Further analysis revealed greater values in the non-stimulated side of group A as compared to the stimulated side of B and both sides of C. Furthermore, motoneurons located on the non-stimulated side of group B were significantly different from the stimulated side of group C.
3.3 Arborization area of dendrites
The ANOVA revealed a main effect of side of stimulation F(1,23)= 28.3, p= 0.00002, and group F(2,23)= 9.84, p= 0.0009, but failed to detect any interaction between side of electrical stimulation and group F(2,23)= 0.01, p= 0.98. The post hoc analysis showed a trend for significance in the within comparison of group A (p= 0.09) and B (p= 0.09), but not in C (p= 0.34). Furthermore, it revealed that the non-stimulated side of group A had greater arborization area of dendrites than the stimulated side of group B, and both sides of group C, respectively. Group A (8414.00 ± 587.10 μm2 for non-stimulated and 6166.62 ± 510.52 μm2 for stimulated), Group B (7730.00 ± 562.56 μm2 for non-stimulated and 5673.62 ± 505.10 μm2 for stimulated), and Group C (5839.96 ± 657.80 μm2 for non-stimulated vs. 3670.37 ± 505.10 μm2 for stimulated).
Overall, the results showed that unilateral stimulation of the somatomotor branch of the pelvic nerve affected the staining of the Pc motoneurons. The total number of assessed motoneurons was smaller in group C (147 for the non-stimulated side and 106 for stimulated side) as compared to groups A (387 for the non-stimulated side and 329 for the stimulated side) and B (329 for the non-stimulated side and 404 for the stimulated side).
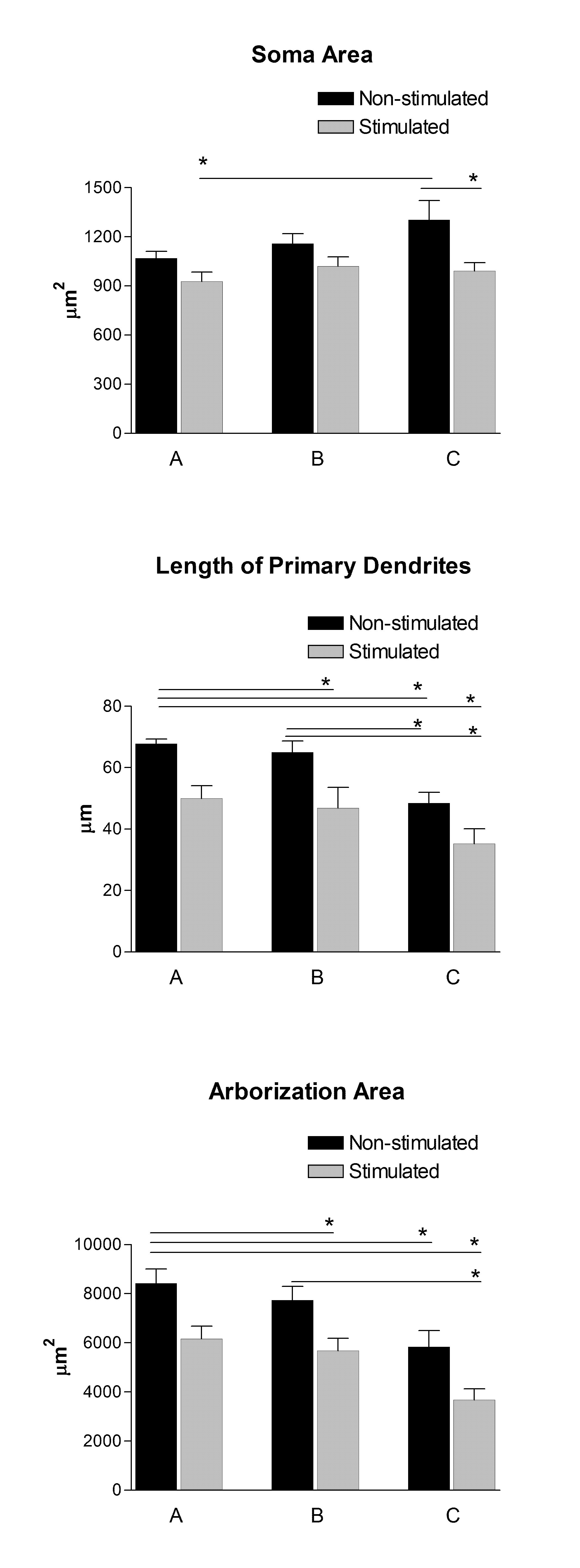
Figure 2. Graphical representations of main morphometric features of Pubococcygeus muscle motoneurons: group A) immediately after the injection of WGA-HRP; group B) 15 min before the sacrifice, and group C) a combination of A+B (i.e., stimulation after injection of the tracer and before perfusion of the rat). Given that electrical stimulation was applied exclusively in the left side of each animal, the right side (non-stimulated) was used as a within-groups comparison to assess the effects of both ipsilateral and contralateral electrical stimulation on the motoneurons staining. Bars represent mean ± SEM in each group; (*) indicates significant differences, P< 0.05.
The data in the present study demonstrate that electrical stimulation of the somatomotor branch of the pelvic nerve produced a reduction of the WGA-HRP labeling in the motoneurons that innervate to the Pc muscle, located in the lumbar-sacral segments of the spinal cord in male rats. Such reduction is observed primarily in neurons located ipsilaterally to the stimulated side, but also in those located contralaterally. In addition, our method allowed us to compare the effects of electrical stimulation that occurred at three different conditions: (A) immediately after the injection of WGA-HRP; (B) 48 h after the injection; and (C) with a combination of methods A and B. Accordingly, the motoneurons located on the ipsilateral side of animals that received electrical stimulation twice (group C) showed the lower values of labeling, which indicates that in our model electrical stimulation affected WGA-HRP labeling in Pc motoneurons. Contrasting our findings, Nishino and colleagues described that electrical stimulation increases HRP labeling as observed in motoneurons of the gastrocnemius muscle.19 Specifically, they demonstrated that in rats, stimulation of the sciatic nerve with 10 Hz 0.1 ms 5V during 1 h before perfusion (higher and longer intensity than in our study) increased the number of gastrocnemius motoneurons labeled with HRP by up to 250 % above the mean number of the control non-stimulated group. A limitation of that study, however, was that they assessed exclusively the number of motoneurons labeled, but not their morphometric characteristics more detailed as soma size and length of dendrites. This study suggested that retrograde transport of HRP in the gastronecmius-sciatic system depends on the activity of both nerve and muscle. It decreases when nerve and muscle activity is low and increases when their activity is high.
In other study, Sato and colleagues20 reported that HRP retrograde labeling of the medial gastrocnemius (MG) motoneurons did not increase by electrical stimulation of the sciatic nerve after the injection of the tracer in the MG muscle bilaterally of rat. In fact, they reported that the number of labeled motoneurons located in the stimulated side was similar (except in the stimulated group early) as compared to the control non-stimulated side in the same individuals.20 They applied electrical stimulation at different periods based on the potential rate of transport of HRP within the neuron. For example, 14 h after injection, the cell bodies began to be observed and 24 h later the labeling intensity of the tracer reached its maximum. Accordingly, it was suggested that electrical stimulation that occurred immediately after injection would affect HRP uptake at the nerve terminal, whereas electrical stimulation 8-16 h and 18-24 h after injection would affect the axonal transport and the process of inactivation of HRP within the soma, respectively. In addition, electrical stimulation was applied at least for 5-10 h in each distinct group, which represents more stimulation in Nishino’s study19 (1 h) or our present study (15 min). Sato and colleagues20 reported that such amount of stimulation during the middle (8-16 h) and late (18-24 h) periods did not affect the numbers of labeled cells. However, when the stimulation was applied immediately after injecting HRP, it produced a significant reduction of labeled cells as compared to the contralateral non-stimulated side. Thus, they suggested that electrical stimulation affected HRP uptake at the nerve terminals, but had no effect on retrograde axonal transport nor had an effect on inactivation of HRP in the soma. In addition, a study conducted by Kanda and colleagues21 observed the effects of blocking nerve conduction on HRP labeling in gastronecmius motoneurons. In their study, the blocking conduction was confirmed by EMG monitoring. Thus, the blocking conduction with tetrodotoxin was reconfirmed 24-48 h after HRP injection into the MG, which is about the required time for the tracer to reach the soma and to be detected. During this study, there were no significant differences in the number of labeled hindlimb motoneurons between the blocked and intact sides of rats. As proposal of this effect, they suggested that in the blocked side may had affected the taking up of HRP at the nerve terminals, but the degradation velocity could be lower in the blocked side as compared to the intact side, which would cancel out any potential difference in labeling. There are several limitations to the previous studies carried out by these authors. For example, the previous studies used old20, 21 and young19 rats in comparison to our study where used adult rats. It has been reported that the penile motoneuron number did not change over time, even with very advanced age. In contrast, the penile muscles and their innervating motoneurons underwent atrophy, with muscle weight and neuron dendritic length declining to less than 50% of young adult levels.27
We hypothesized that the axonal transport of substances in old and young rats is unlike to the axonal transport in adult rats. Hence, our present results are different to the results of previous studies. Moreover, the previous studies used HRP alone and not combined with wheat germ agglutining (WGA) as in our present study. It has been shown that HRP alone is transported more slowly than when combined with WGA.22 In addition, these studies differed in the amount of time allowed for the HRP to be transported since the moment of injection. Specifically, it has been shown that the appropriate time for the tracer to travel from the axon terminals to the soma of motoneurons that innervate to pelvic muscles is about 48 hrs.4, 15, 23-24 Consequently, it is possible that less time as in Nishino’s19 and in Sato’s20 work, plus the use of HRP alone may have not been sufficient for the tracer to reach the soma and dendrites of motoneurons that innervate hindlimb muscles. Unfortunately, they did not consider more variables to assess intracellular distribution of the tracer.
Nevertheless, the data of Nishino and colleagues,19 and Sato and colleagues, suggested two different effects of electrical stimulation. Initially, electrical stimulation applied immediately after injecting HRP appears to diminish its uptake at the nerve terminals. However, when applied several hours later just before perfusion it appears to increase HRP arrival to the soma.
Our results suggest that in all three groups, the soma area for the stimulated side was very similar. In other words, it appears that there is a centripetal motion towards the soma produced by the electrical stimulation. Accordingly, once the HRP is inside the axon and is being transported retrogradely, its arrival to the soma can be accelerated by electrical stimulation. However, in a very similar manner, the HRP that has passed beyond the soma, i.e. that has reached the primary and secondary dendrites, is also brought back to the soma with given electrical stimulation. It is worth noting, that in our present study we used different duration and intensity stimuli and the time of stimulation was only applied for 15 min and these previous studies applied several hours of stimulation. Additionally, we analyzed motoneurons of a pelvic muscle and the previous studies evaluated hindlimb motoneurons.
On the other hand, in previous studies we have shown that the HRP axonal transport of Pc motoneurons is influenced by PVN oxytocin,15 therefore, stimulated pelvic nerve could increase the discharge of this peptide, or any other brain transmitter that is normally released on motoneurons, and the stimulated nerve also could activate both dorsal and motor roots, besides of the supraspinal circuits. Consequently, as a “disinhibition mechanism” the axonal transport of substances is accelerated from dendrites to the soma and affecting part of substances flow from soma to the axon.
Alternatively, other possibility to explain the reduction of labeling could involve increased trans-neuronal transport at the level of the synapses following electrical stimulation, since it has been demonstrated that synaptic activity increase the trans-neuronal transport of WGA-HRP and consequently more of the tracer can be transported out of the stimulated motoneuron population.28-29 However, it has also been shown that free HRP is transported via anterograde, retrograde or transganglionic, but rarely transneuronally.22, 25-26 Given that HRP is an exogenous molecule that has no importance to neural metabolism, the transcellular transfer of this substance would not influence of trophic way of one neuron upon another. This idea may explain why its transneuronal transport is unlikely to happen in Pc motoneurons. Thus, we discard the idea that in our results the tracer had been transported toward another population of neurons, and consequently, the motoneurons had been observed smaller.
Our interpretation suggests that an increase in the electrical activity of motoneurons results in an intradendritic and intraxonal movement (centripetal movement) increased to concentrate substances in the soma. It could represent that several cytoplasmic elements retrogradly transported by electrical stimuli would produce trophic effects or would contribute to an increase for recycling, degradation or transferring of synaptic vesicles and cytoskeleton components into the cell body.
This research was supported by the Consejo Nacional de Ciencia y Tecnología (CONACYT)-Mexico, Grant No. 33124-N. (JM), fellowship 170664 (MCA), and Grant PROMEP/103.5/07/2753 (PTC-244, CAP).
- Lucio RA, Manzo J, Martinez-Gomez M, Sachs BD, Pacheco P. Participation of pelvic nerve branches in male rat copulatory behavior. Physiol Behav 1994 55: 241-246.
- McKenna KE, Nadelhaft I. The organization of the pudendal nerve in the male and female rat. J Comp Neurol 1986 248: 532-549.
- Manzo J, Esquivel A, Hernandez ME, Carrillo P, Martinez-Gomez M, Pacheco P. The role of pubococcygeus muscle in urinary continence in the male rat. J Urol 1997 157: 2402-2406.
- Manzo J, Nicolas L, Hernandez ME, Cruz MR, Carrillo P, Pacheco P. Spinal organization and steroid sensitivity of motoneurons innervating the pubococcygeus muscle in the male rat. J Comp Neurol 1999 409: 358-368.
- Cuevas E, Camacho M, Alvarado M, Hudson R, Pacheco P. Participation of estradiol and progesterone in the retrograde labeling of pubococcygeus motoneurons of the female rat. Neurosci 2006 140: 1435-1442.
- MacLusky NJ, Clark CR, Shanabrough M, Naftolin F. Metabolism and binding of androgens in the spinal cord of the rat. Brain Res 1987 422: 83-91.
- Rousselot P, Papadopoulos G, Merighi A, Poulain DA, Theodosis DT. Oxytocinergic innervation of the rat spinal cord. An electron microscopic study. Brain Res 1990 529: 178-184.
- Swanson LW, McKellar S. The distribution of oxytocin- and neurophysin-stained fibers in the spinal cord of the rat and monkey. J Comp Neurol 1979 188: 87-106.
- Wagner CK, Clemens LG. Projections of the paraventricular nucleus of the hypothalamus to the sexually dimorphic lumbosacral region of the spinal cord. Brain Res 1991 539: 254-262.
- Witt DM, Insel TR. Increased Fos expression in oxytocin neurons following masculine sexual behavior. J Neuroendocrinol 1994 6: 13-18.
- Liu YC, Salamone JD, Sachs BD. Impaired sexual response after lesions of the paraventricular nucleus of the hypothalamus in male rats. Behav Neurosci 1997 111: 1361-1367.
- Tang Y, Rampin O, Calas A, Facchinetti P, Giuliano F. Oxytocinergic and serotonergic innervation of identified lumbosacral nuclei controlling penile erection in the male rat. Neurosci 1998 82: 241-254.
- Veronneau-Longueville F, Rampin O, Freund-Mercier MJ, Tang Y, Calas A, Marson L, McKenna KE, Stoeckel ME, Benoit G, Giuliano F. Oxytocinergic innervation of autonomic nuclei controlling penile erection in the rat. Neurosci 1999 93: 1437-1447.
- Wagner CK, Sisk CL, Clemens LG. Neurons in the paraventricular nucleus of the hypothalamus that project to the sexually dimorphic lower lumbar spinal cord concentrate 3H-estradiol in the male rat. J Neuroendocrinol 1993 5: 545-551.
- Perez CA, Concha A, Hernandez ME, Manzo J. Influence of the paraventricular nucleus and oxytocin on the retrograde stain of pubococcygeus muscle motoneurons in male rats. Brain Res 2005 1041: 11-18.
- Argiolas A, Melis MR. Central control of penile erection: role of the paraventricular nucleus of the hypothalamus. Prog Neurobiol 2005 76: 1-21.
- Tribollet E, Barberis C, Arsenijevic Y. Distribution of vasopressin and oxytocin receptors in the rat spinal cord: sex-related differences and effect of castration in pudendal motor nuclei. Neurosci 1997 78: 499-509.
- Sasaki M, Arnold AP. Androgenic regulation of dendritic trees of motoneurons in the spinal nucleus of the bulbocavernosus: reconstruction after intracellular iontophoresis of horseradish peroxidase. J Comp Neurol 1991 308: 11-27.
- Nishino H, Ono T, Sasaki K, Nishino A, Muramoto K. Retrograde transport of horseradish peroxidase in sciatic nerve of rats and dystrophy mice. Neurosci Lett 1979 14: 1-6.
- Sato H, Kanda K, Hashizume K, Yamada J. Nerve stimulation does not necessarily enhance retrograde HRP labeling of rat motoneuron. Neurosci Lett 1989 107: 81-84.
- Kanda K, Sato H, Hashizume K, Yamada J. The effects of blocking nerve conduction on retrograde HRP labeling of rat motoneuron. Neurosci Lett 1989 99: 153-156.
- van der Want JJ, Klooster J, Cardozo BN, de Weerd H, Liem RS. Tract-tracing in the nervous system of vertebrates using horseradish peroxidase and its conjugates: tracers, chromogens and stabilization for light and electron microscopy. Brain Res Brain Res. Protoc 1997 1: 269-279.
- Kurz EM, Sengelaub DR, Arnold AP. Androgens regulate the dendritic length of mammalian motoneurons in adulthood. Sci 1986 232: 395-398.
- Sengelaub DR, Arnold AP. Hormonal control of neuron number in sexually dimorphic spinal nuclei of the rat: I. Testosterone-regulated death in the dorsolateral nucleus. J Comp Neurol 1989 280: 622-629.
- Mesulam MM. Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: a non-carcinogenic blue reaction product with superior sensitivity for visualizing neural afferents and efferents. J Histochem. Cytochem 1978 26: 106-117.
- Mesulam MM, Rosene DL. Sensitivity in horseradish peroxidase neurohistochemistry: a comparative and quantitative study of nine methods. J Histochem Cytochem 1979 27: 763-773.
- Fargo KN, Iwema CL, Clark-Phelps MC, Sengelaub DR. Exogenous testosterone reverses age-related atrophy in a spinal neuromuscular system. Horm Behav 2007 51: 20-30.
- Alstermark B, Kummel H. Transneuronal transport of wheat germ agglutinin conjugated horseradish peroxidase into last order spinal interneurones projecting to acromio- and spinodeltoideus motoneurones in the cat. 1. Location of labelled interneurones and influence of synaptic activity on the transneuronal transport. Exp Brain Res 1990 80: 83-95.
- Jankowska E, Skoog B. Labelling of midlumbar neurones projecting to cat hindlimb motoneurones by transneuronal transport of a horseradish peroxidase conjugate. Neurosci Lett 1986 71: 163-168.
| Recibido: 9 de septiembre de 2010 | Aceptado: 23 de septiembre de 2010 |
Corresponding Author:
PhD. Cesar Antonio Perez Estudillo, Programa de Neurobiología, Universidad Veracruzana, Xalapa, Ver., Tel.: (228) 841-8900 Ext. 13609, email: cesperez@uv.mx
Este es un artículo de libre acceso distribuido bajo los términos de la licencia de Creative Commons, (http://creativecommons.org/licenses/by-nc/3.0), que permite el uso no comercial, distribución y reproducción en algún medio, siempre que la obra original sea debidamente citada.

