Characterization of four types of tail abnormalities in rats treated prenatally with valproic acid
- Inicio
- Comité Editorial
- Lineamientos
- Carta de Cesión de Derechos
- Información Legal
- Acerca de la Revista
- Bases de Datos
- Contacto
- ISSN 2007-3054
- Centro de Investigaciones Cerebrales
Universidad Veracruzana
Saft P1,2, Toledo-Cardenas R1, Coria-Avila GA1, Perez-Pouchoulen M1, Brug B1, Hernandez ME1, Manzo J1*
1Centro de Investigaciones Cerebrales, Universidad Veracruzana, Xalapa, Ver. México 2Posgrado en Neuroetología, Universidad Veracruzana, Xalapa, Ver, México.
Resumen/Abstract
Introducción
Material y métodos
Resultados
Conclusión
Agradecimientos
Referencias
Correspondencia
El ácido valpróico (AVP) es un fármaco anticonvulsivo usado principalmente para el tratamiento de la epilepsia, trastorno bipolar y esquizofrenia. Se ha demostrado que el AVP es un teratógeno potente que causa defectos de nacimiento y malformaciones. Así mismo, hay informes que sugieren una relación entre el uso de AVP durante el embarazo y un aumento de la incidencia de niños con trastornos neurológicos tales como el espectro autista. En los seres humanos, la exposición prenatal a la AVP produce malformaciones que sugieren un defecto durante el cierre del tubo neural, como es el caso de la espina bífida, enfermedades del corazón, defectos de las extremidades, anomalías craneofaciales, así como anomalías genitales. En el presente trabajo se describen cuatro anormalidades de la cola en ratas tratadas prenatalmente con ácido valpróico. Este tratamiento se ha utilizado como modelo animal para el estudio de rasgos autistas. Estas malformaciones pudieran estar asociadas con algún daño en el sistema nervioso, sin embargo se requieren más estudios para correlacionar cada anormalidad de la cola con el tipo de alteración neural.
Palabras clave: Teratógeno, Ácido valpróico, Modelo animal, Malformaciones, defectos del tubo neural.
Valproic acid (VPA) is an anticonvulsant drug used mainly for the treatment of epilepsy, bipolar disorder and schizophrenia. The VPA has been shown to be a potent teratogen that causes birth defects and malformations. Likewise, there are reports that suggest a relationship between the use of VPA during pregnancy and an increased incidence of children with neurological disorders such as autism. In humans, prenatal exposure to VPA produces malformations including dimorphic facial features suggestive of a lesion in the neural tube, as in the case of spina bifida, heart disease, limb defects and craniofacial anomalies as well as genital abnormalities. Herein we describe four tail abnormalities found in rats treated prenatally with valproic acid, which has been used as an animal model for the study of autistic features. These malformations may be associated with neural damage, but further studies are needed in order to correlate each tail abnormality with the kind of neural alteration.
Key words: Teratogen, Valproic acid, Animal model, Malformations, Neural tube defects.
Valproic acid (VPA) was first synthesized in 1882 by B. S.Burton,1 and is commonly used as both an antiepileptic drug and in some cases to treat personality disorders such as schizophrenia and bipolar disorder, or migraine.2 Its name is derived from the original purification of Valeriana officinalis, which is commonly used to treat insomnia and anxiety.3,4 Shortly after VPA was out in the market, it was demonstrated to cause various malformations in newborns of mothers that were administered VPA during pregnancy. A recent study revealed the association of the treatment with anticonvulsants with at least 14 types of malformations.5 Interestingly, treatment with VPA caused significantly more malformations as compared to carbamazepine, the main anticonvulsant used in humans for the treatment of seizures. These types of malformations have been grouped in a fairly specific embryopathy called fetal valproate syndrome.6 The percentages of malformations in humans are: 12.7% spina bifida, 2.5% atrial septal defect, 5.2% cleft palate, 4.8% hipospadia, 2.2% plolidactilea, 6.8% craniosynostosis.5 Children with prenatal exposure to VPA showed malformations similar to those exposed to thalidomide in utero, but with a reduced severity of the symptoms. These included abnormal features indicative of an injury at the time of neural tube closure, such as heart disease, craniofacial abnormalities, genital abnormalities and limb defects,7 and spina bifida which is the most frequent disorder related to incomplete closing of neural tube.8 Some teratogenic effects of VPA have been also been reported in animals. For example, mice exposed in utero to VPA can develop cardiovascular malformations,9,10 limb defects,11,12 skeletal malformations13 and cerebellar abnormalities such as significantly fewer Purkinje cells in the cerebellar vermis.14 In addition to its teratogenic effects, the use of VPA in pregnant women has been associated with a higher incidence of autism in the offspring.14,15,16,17 A recent study reported that 60% of children who were prenatally exposed to VPA had more than two autistic characteristics but only 11.7% of them met the diagnostic criteria of the autistic spectrum.16 To our knowledge and despite the information presented above, physical malformations in the rat exposed to VPA in utero remain undescribed. Therefore, in the present study we assessed the teratogenic effects of VPA upon the physical condition of the rat body.
Animals
A total of 6 adult females and 6 males, of healthy Wistar rats were used under laboratory conditions. Inverted cycle 12/12h light/dark cycle, (lights off at 08:00 h), food (Rodent Diet, Rismart nu3-225) and fresh water were provided ad libitum.
We determined the late proestrus of every female rat through vaginal cytology to establish the moment in which females were receptive and able to copulate with a male. The couple was left undisturbed for a whole day and then females were isolated in single home cages with a thin layer of aspen chip. The day of isolation was considered as day 1 of gestation. Pregnant rats received a single intraperitoneal injection of 600mg/kg of sodium valproate (NaVPA) on gestational day 12.18 The expected day of delivery animals were monitored and pups were identified by sex and grown until postnatal day 21. Tail malformations were reported until day 21 when it was easy to classify them.
All procedures involving animals were reviewed by and conducted in compliance with the guidelines of Centro de Investigaciones Cerebrales, Universidad Veracruzana, Xalapa, Mexico, based on the standard of Official Mexican Norm NOM-062-ZOO-1999.
A total of 72 pups whose mothers were treated with VPA were obtained from the 6 litters, in which 25 were observed with a tail abnormality. Hence, 34% of animals showed abnormalities. We also did not find any other body malformation besides tail (Figure 1).
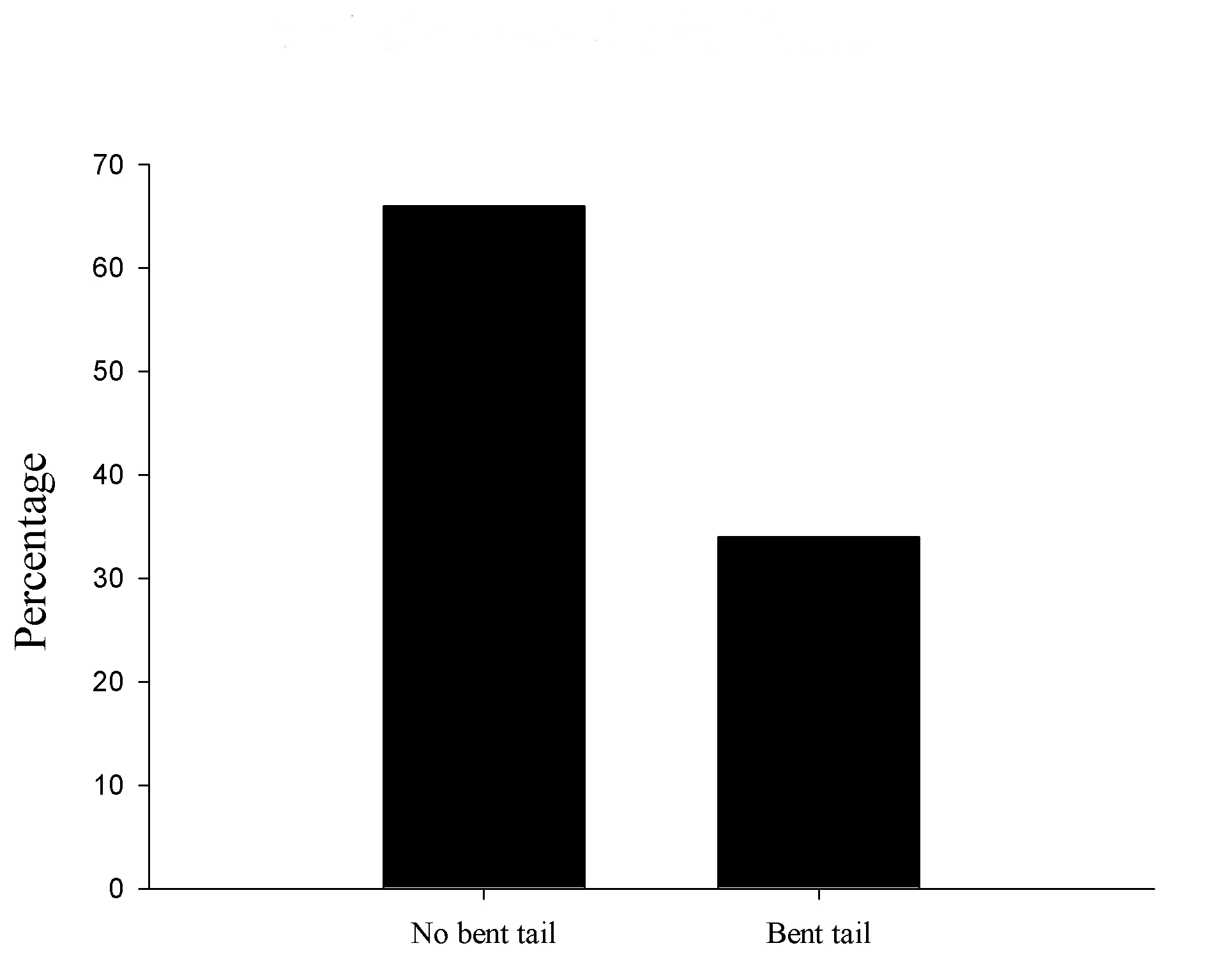
Figure 1. VPA abnormalities in tail incidence. Percentage of bent tail found in treated animals.
Tail description
Four types of tail malformations were observed and each tail type was established according to the distance from the crooked point in the tale to the rat´s body, so the tales were divided in 3 sections: 1) proximal, 2) medial, 3) distal.
Sex differences
We found 16 male and 9 female pups with tail abnormalities out of the 25 total tail bended pups. The proportion was 64% and 36% respectively (Figure 2).
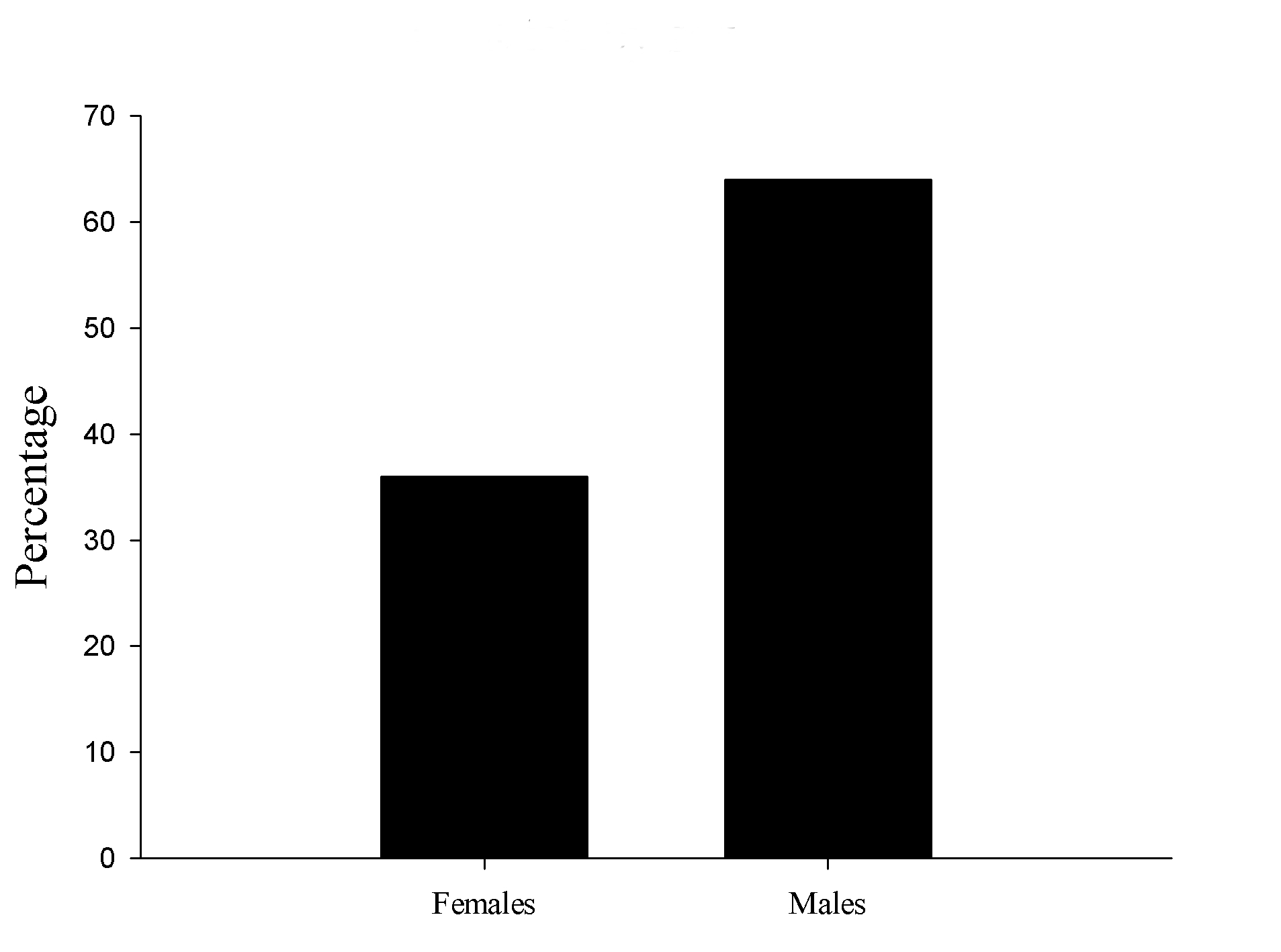
Figure 2. Bent tails by sex. Crooked tails proportion between males and females.
Tail classification
The tail abnormalities were divided in 4 categories according with the features of the length curve and bending shape tail. The categories were: Short tail (A), simple bent in the middle (B), short and bent tail (C), double flexure tail (D) (Figure 3).
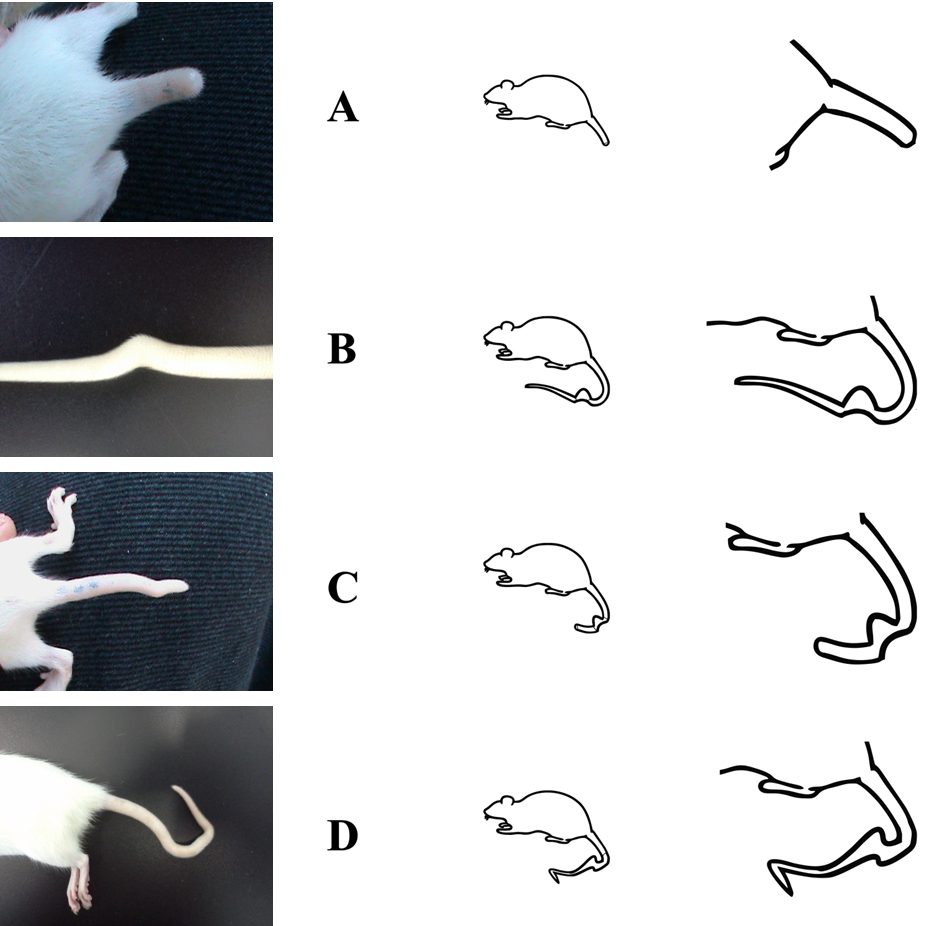
Figure 3. Categories of bent tails. A Short tail, B simple bent in the middle, C short and bent tail, D double flexure tail.
The most frequent feature found were simple bent in 18 pups, 11 males and 7 females; the short tale was observed in 2 males and no female with this malformation. The short and bent tail features were found in 2 males. In the double flexure tail we detected 2 females and only one male (Figure 4).
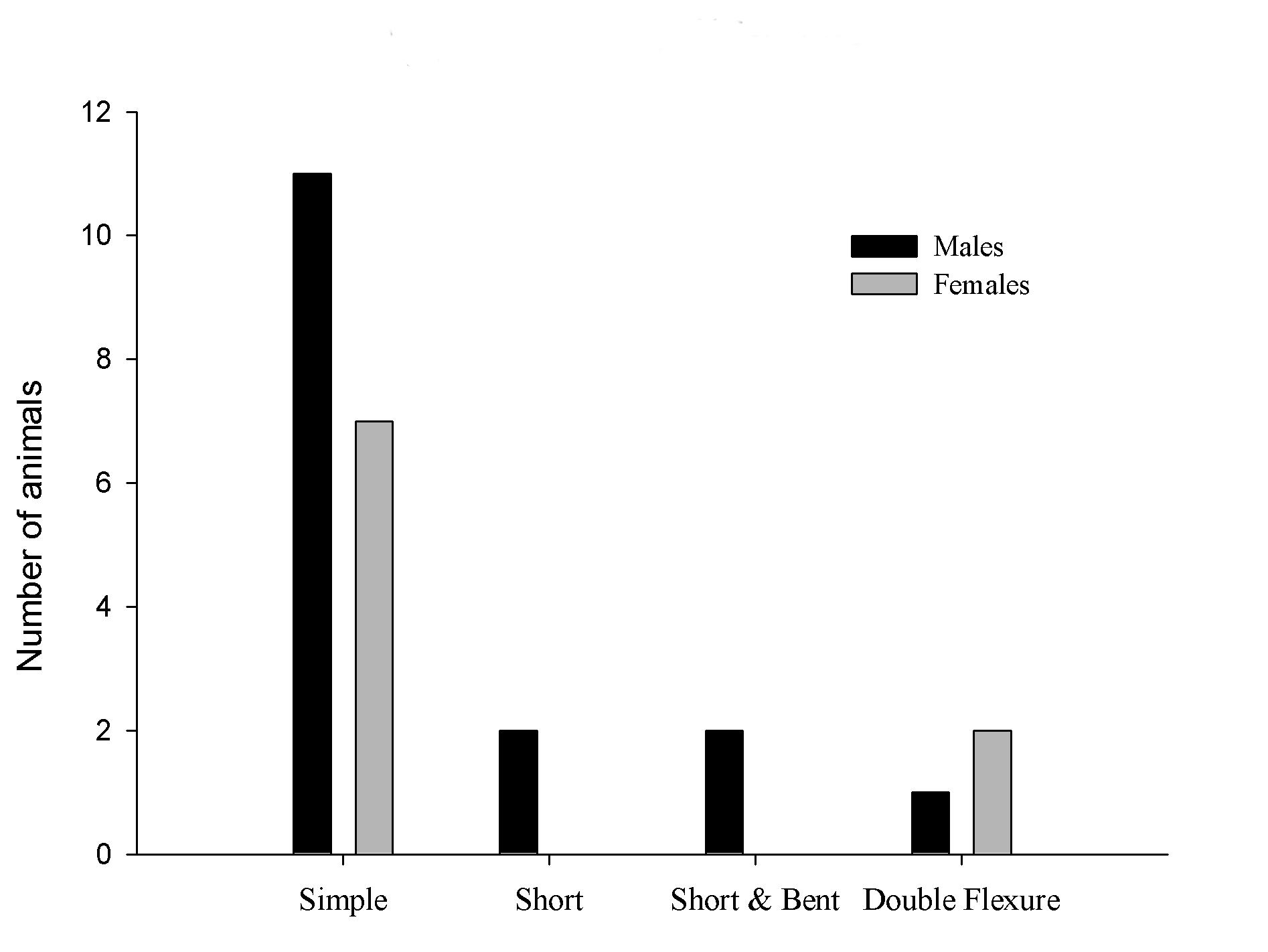
Figure 4. Types of tail abnormalities by sex.
The results confirm that prenatal exposure to valproic acid at critical stages produce teratogenic effects. Specifically, abnormal tail were classified into four groups, the tail as A, B, C, D. The anomalies found in this report illustrate the potent teratogenic effect of valproic acid in these prenatally treated animals. Considering that the method used in this study was first reported as an animal model of autism,8 a disorder with a frequency ratio of 4:1 between human males and females,19 in this sense it is interesting is that in general the found abnormalities were more frequent in males than in females, so may be due to a protective mechanism against VPA present in females.20 On other hand, studies about motor behavior level should consider these possible anomalies during test batteries to validate as an animal model of autism, since that abnormalities in organs such as the tail may cause deterioration in functions such as thermoregulation, as the tail of the rat is a body heat loss; this is due to its length and width, in addition, it lacks skin and nutrients and has not vascularized connections between arteries and veins.21 Another consideration is related to the equilibrium, rats used to distribute weight while climbing or walking through an edge surface.22 Although the mechanisms of teratogenic action of VPA are not yet known23 and the explanation thereof is beyond the present study, our results originate outlook study possible interactions of prenatal treatment with VPA and genes as RORA which has associated with the higher proportion of males and females with autism,24 due to the higher proportion of abnormalities in males than in females (Figure 2). Also allows exploring the hormonal connection because of VPA side effects on steroid hormone levels could be distributed not uniformly across the placenta of embryos, and the differences in the tails shape could be due intrauterine position of the fetus.25 This may have a greater effect in males, as observed in this work. In fact, androgen prenatal exposure affects anogenital distance which is a feature of sexual dimorphism as well fingers length.26,27 As similar process occur with drugs such as cocaine28,29 therefore, future studies could determine whether intrauterine position leads to different levels of prenatal exposure to VPA, like other drugs.
Valproic acid affects normal embryonic development. When an embryo is exposed to VPA at the time of neural tube closure, has a high risk of malformations as twisted and / or bent tail. These malformations are observed in four different types and are more frequent in males. This phenotype could be an indicator of alterations in the central nervous system. These defects must be considered in studies of prenatal treatment of VPA as an animal model of autism, its possible relationship to features of the spectrum and its influence upon assessments for batteries and motor behavioral tests. Further studies are needed to determine the relationship of the anomalies in appendages and structures of the central nervous system.
Centro de Investigaciones Cerebrales Universidad Veracruzana Xalapa México CONACyT scholarship 235940 (PSL) 240116 (BBA) Promep Grant 103.5/07/2753 (RT).
- Gugler R and von Unruh GE. Clinical pharmacokinetics of valproic acid. Clin Pharmacokinet 1980 5: 67-83.
- Wu P, Jiang L, Chen H. Sodium valproate at the therapeutic concentration inhibits the induction but not the maintenance phase of long-term potentiation in rat hippocampal CA1 area. Biochem Bioph Res Co 2010 391: 582-586.
- Kostrouchova M, Kostrouch Z, Kostrouchová M. Valproic acid, a molecular lead to multiple regulatory pathways. Folia Biol-Praha 2007 53: 37.
- Kinrys G, Pollack MH, Simon NM, Worthington JJ, Nardi AE, Versiani M. Valproic acid for the treatment of social anxiety disorder. Int Clin Psychopharm 2003 18: 169- 172.
- Jentink J, Loane MA, Dolk H, Barisic I, Garne E, Morris JK, de Jong-van den Berg LT. Valproic acid monotherapy in pregnancy and major congenital malformations. New Engl J Med 2010 362: 2185-2193.
- Pardal-Fernández JM, Carrascosa-Romero MC, Rodríguez-Vázquez M, Marco-Giner J, Martínez-Gutiérrez A. Embriopatía por ácido valproico con malformaciones graves del sistema nervioso central. Rev Neurología 2006 42: 336- 340.
- Rodier PM, Ingram JL, Tisdale B, Croog VJ. Linking etiologies in humans and animal models: studies of autism. Reprod Toxicol 1997 11: 417-422.
- Koren G, Nava-Ocampo AA, Moretti ME, Sussman R, Nulman I. Major malformations with valproic acid. Can Fam Physician 2006 52: 441-442.
- Sonoda T, Ohdo S, Ohba KI, Okishima T, Hayakawa K. Sodium valproate‐induced cardiovascular abnormalities in the Jcl: ICR mouse fetus: Peak sensitivity of gestational day and dose‐dependent effect. Teratology 1993 48: 127-132.
- Wu G, Nan C, Rollo JC, Huang X, Tian J. Research Sodium valproate-induced congenital cardiac abnormalities in mice are associated with the inhibition of histone deacetylase. Biomed Sci 2010 17: 16-22.
- Menegola E, Broccia ML, Nau H, Prati M, Ricolfi R, Giavini E. Teratogenic effects of sodium valproate in mice and rats at midgestation and at term. Teratogen Carcin Mut 1996 16: 97-108.
- Faiella A, Wernig M, Consalez GG, Hostick U, Hofmann C, Hustert E, Nadeau JH. A mouse model for valproate teratogenicity: parental effects, homeotic transformations, and altered HOX expression. Hum Mol Genet 2000 9: 227-236.
- Downing C, Biers J, Larson C, Kimball A, Wright H, Ishii T, Johnson T. Genetic and maternal effects on valproic acid teratogenesis in C57BL/6J and DBA/2J mice. Toxicol Sci 2010 116: 632-639.
- Ingram JL, Peckham SM, Tisdale B, Rodier PM. Prenatal exposure of rats to valproic acid reproduces the cerebellar anomalies associated with autism. Neurotoxicol Teratol 2000 22: 319-24.
- Rodier PM, Ingram JL, Tisdale B, Nelson S, Romano J. Embryological origin for autism: developmental anomalies of the cranial nerve motor nuclei. J Comp Neurol 1996 370: 247-261.
- Markram H, Rinaldi T, Markram K. The intense world syndrome–an alternative hypothesis for autism. Front Neurosci 2007 1: 77.
- Markram K, Rinaldi T, La Mendola D, Sandi C, Markram H. Abnormal fear conditioning and amygdala processing in an animal model of autism. Neuropsychopharmacology 2007 33: 901-912.
- Schneider T, Przewłocki R. Behavioral alterations in rats prenatally exposed to valproic acid: animal model of autism. Neuropsychopharmacology 2004 30: 80-89.
- Baron-Cohen S. The extreme male brain theory of autism. Trends Cogn Sci 2002 6: 248-254.
- Schneider T, Roman A, Basta-Kaim A, Kubera M, Budziszewska B, Schneider K, Przewłocki R. Gender-specific behavioral and immunological alterations in an animal model of autism induced by prenatal exposure to valproic acid. Psychoneuroendocrino 2008 33: 728-740.
- Vanhoutte G, Verhoye M, Raman E, Roberts M, Linden A. In‐vivo non‐invasive study of the thermoregulatory function of the blood vessels in the rat tail using magnetic resonance angiography. NMR Biomed 2002 15: 263-269.
- Hickman GC. The mammalian tail: a review of functions. Mammal Rev 1979 9: 143-157.
- Tung EW, Winn LM. Valproic acid increases formation of reactive oxygen species and induces apoptosis in postimplantation embryos: a role for oxidative stress in valproic acid-induced neural tube defects. Mol Pharmacol 2011 80: 979-987.
- Sarachana T, Xu M, Wu RC, Hu VW. Sex hormones in autism: androgens and estrogens differentially and reciprocally regulate RORA, a novel candidate gene for autism. PLoS One 2011 6: e17116.
- Barr M, Brent RL. The relation of the uterine vasculature to fetal growth and the intrauterine position effect in rats. Teratology 1970 3: 251-260.
- Yan RH, Bunning M, Wahlsten D, Hurd, PL. Digit Ratio (2D∶ 4D) Differences between 20 Strains of Inbred Mice. 2009 PloS one 4(6), e5801.
- Manning JT, Callow M, Bundred PE. Finger and toe ratios in humans and mice: implications for the aetiology of diseases influenced by HOX genes. Medical Hypotheses 2003 60(3), 340-343.
- Ryan BC, Vandenbergh JG. Intrauterine position effects. Neurosci Biobehav Rev 2002 26(6), 665-678.
- Mitchell ES, Keller Jr RW, Snyder-Keller A. Immediate-early gene expression in concurrent prenatal ethanol-and/or cocaine-exposed rat pups: intrauterine differences in cocaine levels and Fos expression. Dev Brain Res 2002 133: 141-149.
| Recibido: 10 de junio de 2014 | Aceptado: 07 de julio de 2014 |
Correspondencia:
Centro de Investigaciones Cerebrales, Universidad Veracruzana. Avenida Luis Castelazo Ayala s/n Col. Industrial Ánimas. C. P. 91190. Xalapa, Veracruz, Mexico. Phone: +52(228) 8418900 Ext 16308 email: jmanzo@uv.mx
Este es un artículo de libre acceso distribuido bajo los términos de la licencia de Creative Commons, (http://creativecommons.org/licenses/by-nc/3.0), que permite el uso no comercial, distribución y reproducción en algún medio, siempre que la obra original sea debidamente citada.

